What Was The Point Of Creating An Hybrid Flu/RSV Virus?
Did The "Research" Really Add To What Is Known About Viruses?
Digging deeper into the lab research1 which resulted in the creation of an hybrid virus from Influenza A and Respiratory Syncytial Virus, one must at the end ask the question: “What was the point”? What was gained by facilitating the hybridization of two of the most dangerous infectious respiratory pathogens in existence?
From reading the study, the answer appears to be “Not much.”
Despite claiming to be a study of the phenomenon known as “coinfection”, mixing Influenza A virus (IAV) and Respiratory Syncytial Virus (RSV) seems to merely confirm the reality that co-infection happens. That is hardly the sort of investigative payoff that would justify even the accidental creation of a hybrid virus.
Co-Infection Has Long Been Known To Happen
Far from this study being groundbreaking research into the ability of multiple viruses to infect a human host simultaneously, the reality is that co-infection has long been an acknowledge virological phenomenon.
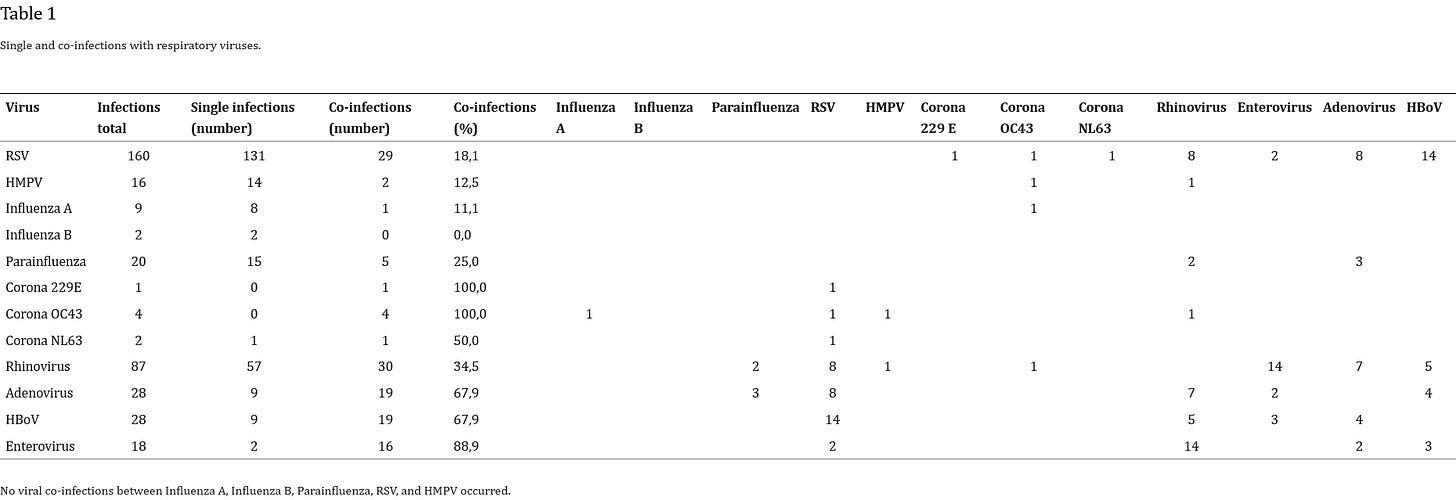
A 2010 study2, published in the Journal of Clinical Virology, of pediatric patients admitted to hospital for lower respiratory tract infections (LRTIs) found a fairly large incidence of coinfection.
Viral single infections were detected in 66% and co-infections in 34% of all viral infections (Table 1 ). Two or more viruses were found in 67 cases (17%). A RSV viral co-infection with human bocavirus was the single most common observation (n = 14) (48%). The majority of the rhinovirus co-infections were in combination with enterovirus (n = 14) (47%). Overall, 30 viral co-infections were in combination with rhinovirus, 22 (73%) of these were dual infections, and 8 (27%) were infections with more than two viruses. Nineteen (68%) of the 28 detected infections with human bocavirus and adenovirus were viral co-infections. No viral co-infection between influenza A, influenza B, parainfluenza, RSV, and HMPV occurred.
The patient population for this study was 404 pediatric patients up to 16 years of age.
Right off the bat, therefore, we have to question the purpose of deliberately mixing Influenza A and Respiratory Syncytial Virus in vitro, inasmuch as the 2010 study suggests that such a pairing of viral infections is at the very least rare. In fact, according to the co-infections found in the 2010 study, Influenza A and Influenza B viruses are the least likely to be part of a co-infection.
For whatever reason, Rhinovirus, Adenovirus, and Human Bocavirus are the pathogens most likely to join RSV in a co-infection scenario, going by the 2010 study.
While this one study is hardly sufficient proof to say that coinfections involving Influenza A or Influenza B do not happen, it does indicate that, if one wishes to study coinfections in a laboratory setting, there are far better candidates for pairing with RSV than Influenza A.
Joanne Haney and her colleagues, in selecting Influenza A and RSV for their research, seem curiously oblivious to the fact that they chose a pairing which the 2010 study indicates is an highly unlikely one to happen in nature.
Here we examined interactions between two commonly co-circulating viruses of clinical importance: IAV and RSV. IAV causes over five million hospitalizations each year and RSV is the leading cause of acute lower respiratory tract infection in children under five years of age. To study virus–virus interactions during coinfection, we infected a cell line derived from human lung (A549), with a mixed inoculum of IAV and RSV, or individual viruses as controls.
This seeming obliviousness is even more curious given their citation of the 2010 study; it is empirical fact that they were not just aware of the study but of its details.
Co-infections do happen. They do not usually happen with Influenza A and RSV.
They Went Out Of Their Way To Facilitate Infection And Hybridization
In what is another potential deviation from what happens naturally when viruses infect cells, Haney and her team went out of their way to ensure not just that infection happened, but that hybridization had ample opportunity to occur. They did this by dosing the culture cells at a high Multiplicity Of Infection level.
Infections were performed at high MOI to facilitate coinfection and recapitulate high MOIs, which are produced in advanced stages of infection (when IAV and RSV foci may come into contact).
The explicit intent of this study was to bring Influenza A and RSV into contact with each other within the same infected cells; they were looking for hybridization.
Bear in mind that the 2010 study referenced above indicates Influenza A and RSV rarely come in to contact with each other in this faction.
While this is not in the same category of Gain-of-Function experiments as the reckless and dangerous molecular manipulations of SARS-CoV-2 performed by both Boston University and Imperial College London, to deliberately cause viruses to join into hybrids still invites the risk that the resulting hybrids will have greater pathogenicity, and so should be viewed through the Gain-of-Function lens—i.e., with extreme suspicion and reflexive disapproval.
By their very nature, such research efforts have no control over the outcome. There is no assurance that a resulting hybrid would not be either more infectious or more lethal.
We know that Joanne Haney and her team succeeded in creating a hybrid virus that had greater capacity than original Influenza A virus to evade antibody-mediated neutralization. We know this because they said so:
As surface glycoproteins determine antigenicity and tropism, and HVPs incorporate glycoproteins of both IAV and RSV, we hypothesized that HVPs would display altered antigenicity. To test this, we first compared the neutralization efficiency of anti-IAV HA antibodies against viruses collected from cells infected with IAV alone, or coinfected with IAV and RSV. As RSV is predominantly cell-associated, we performed neutralization assays using supernatant and cell-associated fractions (Methods). Viruses were also back-titrated to determine infectious titre of the inoculum (Extended Data Fig. 6a,b). Viruses collected from coinfected cells displayed reduced IAV neutralization compared to those collected from single IAV infections (Extended Data Fig. 6c). While the observed differences were not statistically significant, the decrease in neutralization efficiency was more marked in the cell-associated fraction of the coinfected cells (Extended Data Fig. 6c) where only 33% (±27%; mean [±SD]) of IAV was neutralized, suggesting that two-thirds of IAV within this fraction was able to evade antibody-mediated neutralization (Extended Data Fig. 6c).
However, even this is not at all surprising, as a 2015 prospective cohort study3, published in Epidemiology And Infection, found that co-infection with RSV resulted in a more severe and longer lasting infection.
The rate of RSV recovery was lower (i.e. shedding duration increased) by 65% in episodes with co-infection compared to those without (aHR 0·35, 95% CI 0·23–0·51), with a similar result for each virus individually. Detection of infection with any one or more of rhinovirus, adenovirus or coronavirus, in the 2 weeks preceding the start of RSV infection, but not during the RSV episode itself, was associated with a 56% increase in the rate of recovery (i.e. reduced shedding duration) from the RSV infection (aHR 1·56, 95% CI 1·02–2·39). In contrast, RSV episodes associated with detection of other viruses in the 2 weeks prior to and also during the RSV infection were associated with a 52% decrease in the rate of recovery relative to those with no other virus prior to and during the RSV episode (aHR 0·48, 95% CI 0·32–0·73).
Not Just “Hybrid Virus Particles”, But Hybrid Virus
While Joanne Haney and her research team take great pains to speak of “Hybrid Virus Particles”, there is no doubt that they are describing a hybrid virus. They even went so far as to prove the hybrid virus was capable of infection other cells.
Our cryo-ET data showed that HVPs contain IAV and RSV genomes. To determine if they possess infectivity for both viruses, we infected NA-treated cells with virus harvested from single or mixed infections. At 12 hpi, cells were fixed and stained for IAV HA and RSV F and imaged by confocal microscopy. The presence of coinfected cells suggested that both genomes were delivered into the cells simultaneously, likely by HVPs (Fig. 5a,b). This conclusion is based on the facts that 12 hours is not a sufficiently long time to allow extensive cell-to-cell spread by IAV and RSV that may result in coinfection, and because the probability of coinfection by chance was low as the MOI for each virus was approximately 0.01 (based on back-titrations of harvested virus). Examination of coinfected cells using super-resolution microscopy revealed viral IAV HA and RSV F double-positive filaments (Fig. 5b), suggesting that the HVPs can be maintained over virus passage. To establish if HVPs could spread IAV from cell to cell within a population of cells refractory to IAV infection, we infected NA-treated or control cells with virus harvested from IAV single infections or from the cell-associated fraction of mixed infections (the fraction with enriched HVPs). We then applied an overlay to prevent virion diffusion and incubated the cells for 48 hours. As expected, in single IAV infections, abundant IAV-positive cells were observed in non-treated cells (Fig. 5c, top row). In the NA-treated cells, no infection by IAV from single infections was observed (Fig. 5c, middle row), whereas IAV foci consisting of multiple distinct infected cells were observed in NA-treated cells infected with virus from mixed infections (Fig. 5c, bottom row). Notably, IAV-positive foci colocalized with RSV-positive foci (Fig. 5c, bottom row). These results suggests that HVPs can mediate the spread of IAV within a refractory cell population.
A virus hybrid which can infect other cells in a lab culture potentially can infect other cells in another human host. Should it be found that this Frankenvirus creation can be shed and transmitted from one person to another, the possibility exists that this hybrid is already a new pathogen of significant pandemic potential.
Thus there should be no illusions about what the Haney team did—created a new virus by blending Influenza A and RSV in a single virion.
They proved that Influenza A and RSV can join together to form an hybrid.
They proved that the hybrid virus can infect other cells both within the same culture and in different cultures with what amounts a combination of Influenza A and RSV.
These functionalities mean there is more than just “hybrid virus particles”. They created a hybrid virus.
Was Any Of It Necessary?
What did Haney and her team accomplish by this random act of viral creation?
While they tout in the abstract that they identified a previously unknown viral interaction, that really is not true. Yes, the hybridization of Influenza A and RSV was previously unknown, but that is at least in part because, based on the 2010 study, Influenza A and Respiratory Syncytial Virus are an unlikely pairing in naturally occuring disease.
Moreover, Haney and her team acknowledge that various modes of hybridization are not only possible, but to a degree even probable.
At the cellular level, the interactions that determine the outcome of coinfections are unclear. Direct interactions between viruses within coinfected cells can result in changes to viral progeny, including, but not limited to, pseudotyping (incorporation of surface proteins from a different virus) or genomic rearrangements, which may generate novel strains with pandemic potential such as SARS-CoV-2 and pandemic influenza A viruses.
Note that they also acknowledge the pandemic potential of such creations. Despite knowing this, they created the hybrid anyway.
To what end? The extant research—much of it cited by Haney and her research team—has already established much of what Joanne Haney claims as original findings.
The mechanics of an hybridization that is unlikely to occur naturally, given that it is an apparently rare pairing of viruses in a coinfection is an amazingly small benefit for a research project which could quite easily have created the next pathogen to escape from the lab to torment the world with yet more disease.
There simply was no good reason for this research to have been done, and absolutely no reason for it to have taken the form that it did.
One more reason why virologists need to stop playing God with viruses. The world does not need any more instances of Gain-of-Function Russian Roulette.
Haney, Joanne et al. “Coinfection by influenza A virus and respiratory syncytial virus produces hybrid virus particles.” Nature microbiology vol. 7,11 (2022): 1879-1890. doi:10.1038/s41564-022-01242-5
Franz, Anna et al. “Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection.” Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology vol. 48,4 (2010): 239-45. doi:10.1016/j.jcv.2010.05.007
Munywoki, P K et al. “Influence of age, severity of infection, and co-infection on the duration of respiratory syncytial virus (RSV) shedding.” Epidemiology and infection vol. 143,4 (2015): 804-12. doi:10.1017/S0950268814001393




Unethical science does more to damage democracy and liberty than 'misinformation' ever could.
Who's going to stop them? With the way pharma has infiltrated all aspects and levels of academia and public health, it holds powerful sway over our medical existence.
It's going to take some very brave people to reverse this.
Good article hadn't heard of this particular research where a hybrid was created, just have heard the fact that the two viruses could cause a coinfection. Curious as to how you found out about the GOF research?
Your paragraph towards the end perhaps explains the answer to your own question of What Was The Point: "The mechanics of an hybridization that is unlikely to occur naturally, given that it is an apparently rare pairing of viruses in a coinfection is an amazingly small benefit for a research project which could quite easily have created the next pathogen to escape from the lab to torment the world with yet more disease."
Creating the next pathogen to torment the world may have been the intent!!! Linking tomorrow @https://nothingnewunderthesun2016.com/